
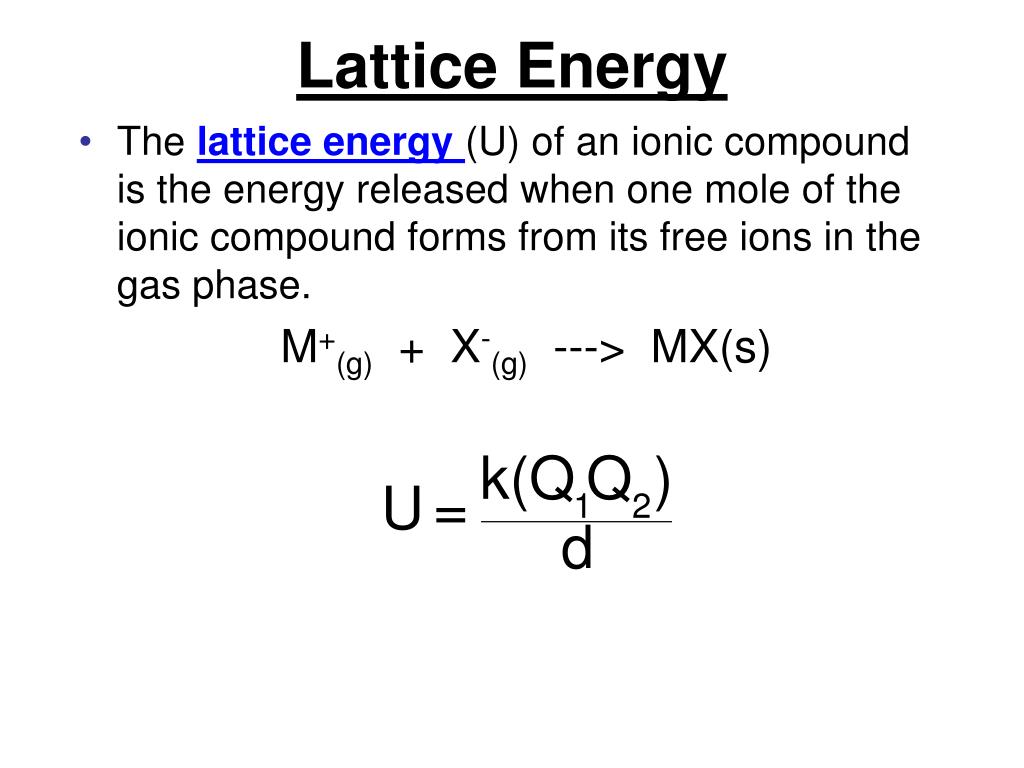
Magnesium oxide is produced by the calcination of magnesium carbonate or magnesium hydroxide. The pure powder of MgO has a relative permittivity inbetween 3.2 to 9.9 k k with an approximate dielectric loss of tan(δ) > 2.16x10 3 at 1kHz. Pure MgO is not conductive and has a high resistance to electric current at room temperature. Because of its stability, MgO is used as a model system for investigating vibrational properties of crystals.

According to evolutionary crystal structure prediction, MgO 2 is thermodynamically stable at pressures above 116 GPa (gigapascals), and a semiconducting suboxide Mg 3O 2 is thermodynamically stable above 500 GPa. While "magnesium oxide" normally refers to MgO, the compound magnesium peroxide MgO 2 is also known. Magnesium oxide was historically known as magnesia alba (literally, the white mineral from Magnesia), to differentiate it from magnesia negra, a black mineral containing what is now known as manganese. Magnesium hydroxide forms in the presence of water (MgO + H 2O → Mg(OH) 2), but it can be reversed by heating it to remove moisture. It has an empirical formula of MgO and consists of a lattice of Mg 2+ ions and O 2− ions held together by ionic bonding. Magnesium oxide ( Mg O), or magnesia, is a white hygroscopic solid mineral that occurs naturally as periclase and is a source of magnesium (see also oxide).


 0 kommentar(er)
0 kommentar(er)
